- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
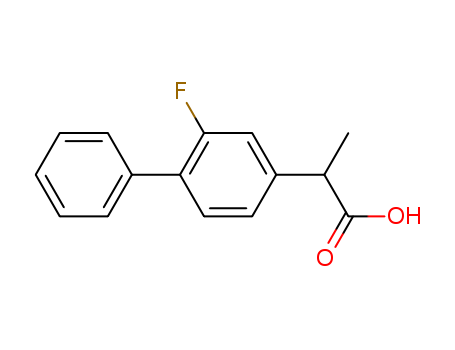
- Flurbiprofen
- 5104-49-4
- C<sub>15</sub>H<sub>13</sub>FO<sub>2</sub>
- 244.265
- white to off-white crystalline solid
Your Location:Home > Products > API > Flurbiprofen

|
Anti-inflammatory analgesics |
Flibanserin , also known as flurbiprofen, flurbiprofen, is a potent Phenylalanine anti-inflammatory and antipyretic analgesics,it can inhibit prostaglandin synthesizing cyclooxygenase to have analgesic, anti-inflammatory and antipyretic effects. Its anti-inflammatory and analgesic effects are 250 times and 50 times of aspirin (also known as acetylsalicylic acid) . The oral absorption is rapid , peak plasma concentration achieves after 1.5 hours , half-life is 3.5 hours, it has wide tissue distribution, PPB is 99.4%, it can compete with drugs having a high plasma protein binding rate to bind plasma protein .it Metabolizes in the liver and becomes flurbiprofen hydroxy and its aldehyde acid conjugates. T1/2 is 3.5 h. Urine and fecal excretion,account for approximately 60% and 40% respectively . Age has no effect on drug metabolism. It is Mainly used for rheumatoid arthritis, rheumatoid arthritis, ankylosing spondylitis, osteoarthritis. It is also used in preventing aphakic cystoid patchy edema After surgical removal of the lens, inhibiting pupillary constrictionsurgery, treatment of inflammation after cataract and trabeculoplasty argon laser eye surgery. It Also applies to pain caused by some other reasons such as trauma, sprains, surgery. |
|
Toxicity |
Non-steroidal anti-inflammatory drug (NSAID) has anti-inflammatory, analgesic and antipyretic effects, toxicity ascending ranking is nabumetone, salsalate, sulindac, diclofenac, ibuprofen, one fabric ibuprofen, aspirin, naproxen, tolmetin, flurbiprofen, piroxicam, a phenoxy ibuprofen, indomethacin, mefenamic acid chlorine. Traditional NSAID medications may be the preferred aspirin, if children in the course of treatment can not tolerate its adverse reactions, use of other non-steroidal anti-inflammatory drugs is taken. a selective COX-2 inhibitor Has been developed, which will replace all traditional NSAID. Selective COX-2 inhibitors which has been listed are nimesulide (Nimeng Shu), rofecoxib (Vioxx), celecoxib (Celebrex), etodolac (Rodin), meloxicam. A recent large-scale, international, multi-center, randomized, double-blind technology, prospective study has shown that selective COX-2 inhibitors have few side effects on the gastrointestinal tract, kidneys, having no significant effect on platelet function,it can be used as drug of choice for early combination therapy of JRA children replacing aspirin . The above information is edited by the lookchem of Tian Ye. |
|
Adverse reactions |
The most common adverse reactions are indigestion, stomach discomfort, occasional headache, skin rash. Peptic ulcer, bronchial asthma patients and pregnant women, lactating women should not take. Other adverse reactions are nausea, diarrhea, abdominal pain, blurred vision, urinary tract infection symptoms, dermatitis. Few have elevated liver transaminases, continuing medication, may develop, or remain unchanged or disappear. Mild tingling and burning sensations and (or) visual disturbances when it is dropped into the eye.because it leads to platelet aggregation and prolongs bleeding time, it is reported that the application of the drug in the eye surgery increases intraocular hemorrhage tendency.in Animal experiments, Flibanserin 50~100 mg/kg, medication for three months, can cause renal papillary necrosis. For Humans,it also has this effect. |
|
production method |
It is obtained by 2-fluoro-linked acetophenone through oxidation, esterification, transesterification, hydrolysis, decarboxylation reaction. |
|
Indications |
Flurbiprofen (Ansaid) is indicated for the treatment of rheumatoid arthritis and osteoarthritis. Its half-life, longer than that of many of the NSAIDs, allows for twice daily dosing.The most common adverse effects of flurbiprofen are similar to those of the other acidic NSAIDs. Flurbiprofen inhibits both COX isoforms about equally. |
|
Manufacturing Process |
A mixture of 3-acetyl-2-fluorobiphenyl, MP 95°C to 96°C, (73.5 g) [prepared from 4.bromo-3-nitroacetophenone (Oelschlage, Ann., 1961, 641, 81) via-4acetyl-2-nitrobiphenyl, MP 106°C to 108°C (Ullman reaction), 4-acetyl-2aminobiphenyl, MP 124°C to 125°C (reduction), and finally the Schiemann reaction], sulfur (17.4 g) and morpholine (87 ml) was refluxed for 16.5 hr, and then the resulting thiomorpholide was hydrolyzed by refluxing with glacial acetic acid (340 ml) concentrated sulfuric acid (54 ml) and water (78 ml) for 24 hr. The cooled solution was diluted with water, and the precipitated crude 2-fluoro-4-biphenylylacetic acid was collected. (A sample was purified by recrystallization to give MP 143°C to 144.5°C; Found (%): C, 73.2; H, 4.8. C14H11FO2 requires C, 73.1; H, 4.8.)A sodium carbonate solution of the crude acetic acid was washed with ether and then acidified with hydrochloric acid; the required acid was isolated via an ether extraction and was esterified by refluxing for 6 hr with ethanol (370 ml) and concentrated sulfuric acid (15 ml). Excess alcohol was distilled, the residue diluted with water and the required ester isolated in ether. Distillation finally gave ethyl 2-fluoro-4-biphenylacetate, BP 134°C to 136°C/0.25 mm.This ester (70g) and diethyl carbonate (250 mg) were stirred at 90°C to 100°C while a solution of sodium ethoxide [from sodium (7.8 g) and ethanol (154 ml)] was added over 1 hr. During addition, ethanol was allowed to distill and after addition distillation was continued until the column heat temperature reached 124°C. After cooling the solution to 90°C, dimethyl sulfate (33 ml) was followed by a further 85 ml of diethyl carbonate. This solution was stirred and refluxed for 1 hr and then, when ice cool, was diluted with water and acetic acid (10 ml). The malonate was isolated in ether and fractionally distilled to yield a fraction boiling at 148°C to 153°C/0.075 mm, identified as the alpha-methyl malonate. This was hydrolyzed by refluxing for 1 hr at 2.5 N sodium hydroxide (350 ml) and alcohol (175 ml), excess alcohol was distilled and the residual suspension of sodium salt was acidified with hydrochloric acidto give a precipitate of the alpha-methyl malonic acid. This was decarboxylated by heating at 180°C to 200°C for 30 minutes and recrystallized from petroleum ether (BP 80°C to 100°C) to give 2-(2-fluoro-4biphenylyl)propionic acid, MP 110°C to 111°C |
|
Therapeutic Function |
Antiinflammatory |
|
Biological Activity |
Potent inhibitor of cyclooxygenase (IC 50 values are 0.1 and 0.4 μ M for inhibition of human COX-1 and COX-2 respectively). Analgesic, anti-inflammatory and antipyretic in vivo . Inhibits tumor cell growth in vitro and in vivo . Also inhibits fibroblast proliferation in vitro . |
|
Pharmacokinetics |
Flurbiprofen is well absorbed after oral administration, with peak plasma levels being attained within 1.5 hours. Food alters the rate of absorption but not the extent of its bioavailability. It is extensively bound to plasma proteins (99%).and has a plasma half-life of 2 to 4 hours. Metabolism is extensive, with 60 to 70% of flurbiprofen and its metabolites being excreted as sulfate and glucuronide conjugates. Flurbiprofen shows some interesting metabolic patterns, with 40 to 47% as the 4′-hydroxy metabolite, 5% as the 3′,4′-dihydroxy metabolite, 20 to 30% as the 3′-hydroxy- 4′-methoxy metabolite, and the remaining 20 to 25% of the drug being excreted unchanged. None of these metabolites demonstrates significant anti-inflammatory activity. |
|
Drug interactions |
Potentially hazardous interactions with other drugs ACE inhibitors and angiotensin-II antagonists: antagonism of hypotensive effect; increased risk of nephrotoxicity and hyperkalaemia. Analgesics: avoid concomitant use with other NSAIDs or aspirin; avoid concomitant use with ketorolac (increased side effects and haemorrhage). Antibacterials: possibly increased risk of convulsions with quinolones. Anticoagulants: effects of coumarins and phenindione enhanced; possibly increased risk of bleeding with heparin, dabigatran and edoxaban - avoid long term use with edoxaban. Antidepressants: increased risk of bleeding with SSRIs or venlafaxine. Antidiabetics: effects of sulphonylureas enhanced. Antiepileptics: possibly enhanced effect of phenytoin. Antivirals: concentration possibly increased by ritonavir; increased risk of haematological toxicity with zidovudine. Ciclosporin: may potentiate nephrotoxicity. Cytotoxics: reduced excretion of methotrexate; increased risk of bleeding with erlotinib. Diuretics: increased risk of nephrotoxicity; antagonism of diuretic effect; hyperkalaemia with potassium-sparing diuretics. Lithium: excretion reduced (risk of lithium toxicity). Pentoxifylline: increased risk of bleeding. Tacrolimus: increased risk of nephrotoxicity |
|
Metabolism |
Flurbiprofen is metabolised mainly by hydroxylation (via the cytochrome P450 isoenzyme CYP2C9) and conjugation in the liver and excreted in the urine. The rate of urinary excretion of flurbiprofen and its two major metabolites ([2-(2-fluoro-4′-hydroxy-4-biphenylyl) propionic acid] and [2-(2-fluoro-3′-hydroxy-4′-methoxy-4-biphenylyl) propionic acid]) in both free and conjugated states is similar for both the oral and rectal routes of administration. |
|
Brand name |
Ansaid (Pharmacia & Upjohn). |
|
General Description |
Flurbiprofen (Ansaid, Ocufen, Froben), is another drug inthis class indicated for both acute and long-term managementof RA and OA but with a more complex mechanism ofaction. Unlike the other drugs in this class, it does not undergochiral inversion (i.e., the conversion of the “inactive”[R]-enantiomer to the active, [S]-enantiomer). Similar to aspirinand other salicylates, both flurbiprofen enantiomersblock COX-2 induction as well as inhibiting the nuclearfactor-κB-mediated polymorphonuclear leukocyte apoptosissignaling; therefore, both enantiomers are believed to contributeequally to its overall anti-inflammatory action.(R)-flurbiprofen is actually a strong clinical candidate forthe treatment of Alzheimer disease, because it has beenshown to reduce Aβ42 production by human cells. |
InChI:InChI=1/C12H4F14/c13-7(9(15,16)17,10(18,19)20)5-1-2-6(4-3-5)8(14,11(21,22)23)12(24,25)26/h1-4H
Cell-bound lipases of dry mycelium of As...
The inclusion behavior of methylated β-c...
Five optically active urea derivatives (...
The lipase-catalysed kinetic resolution ...
The solvent versatility of Chiralpak IB,...
Mycelia of Aspergillus oryzae display hi...
A highly enantioselective synthesis of (...
A novel method for chiral separation of ...
The 2-arylpropionic acid derivatives or ...
A method was developed and validated for...
We report a stereoselective conversion o...
(S)-Flurbiprofen (1) is a nonsteroidal a...
Catalytic deracemization of a-branched a...
The invention relates to the technical f...

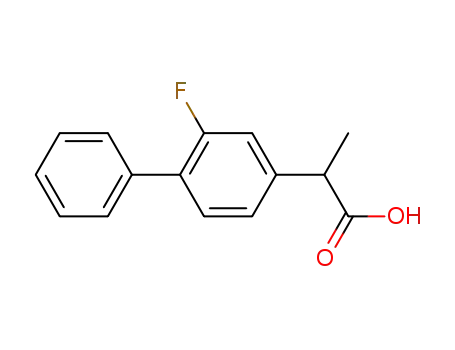
fluorobiprofen

Sucrose
| Conditions | Yield |
|---|---|
|
|
|
|
|
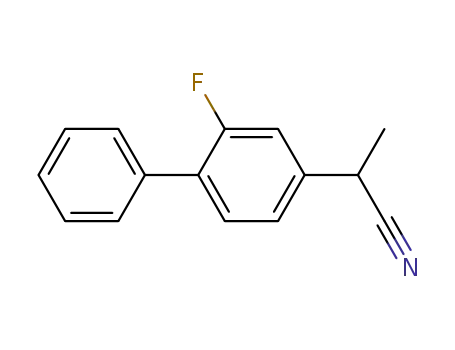
α-methyl-(2-fluoro-4-biphenylyl)acetonitrile


fluorobiprofen
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In ethanol; water; at 110 ℃; for 5h;
|
99% |
|
With sodium hydroxide; In ethanol; water; at 110 ℃; for 7h; Inert atmosphere;
|
84% |
|
α-methyl-(2-fluoro-4-biphenylyl)acetonitrile; With sodium hydroxide; In ethanol; water; for 5h; Inert atmosphere; Reflux;
With sulfuric acid; In ethanol; water; at 20 ℃; Inert atmosphere;
|
82% |
|
With sulfuric acid; In water; at 100 ℃; for 3h;
|
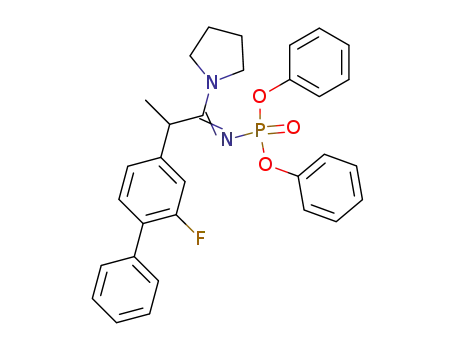
diphenyl N-<2-(2-fluoro-4-biphenylyl)-1-pyrrolidinopropylidene>phosphoramidate
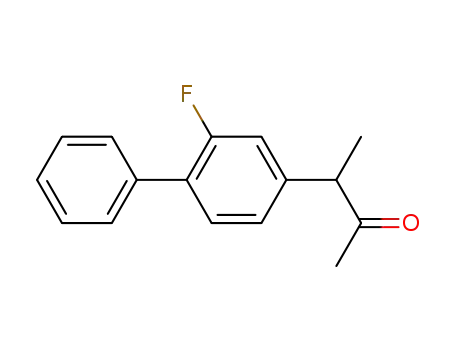
3-(2-fluorobiphenyl-4-yl)butan-2-one
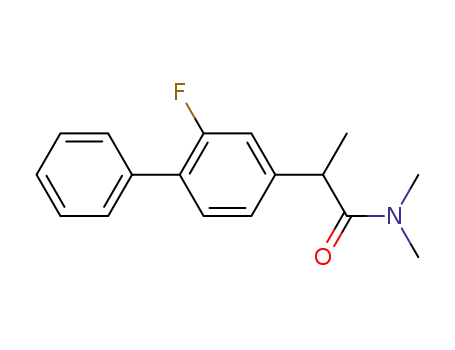
2-(2-Fluoro-biphenyl-4-yl)-N,N-dimethyl-propionamide
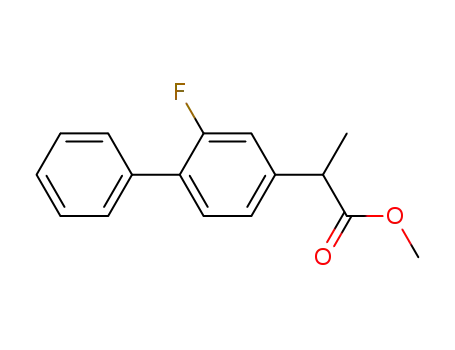
methyl 2-(2-fluoro-4-biphenylyl)propionate
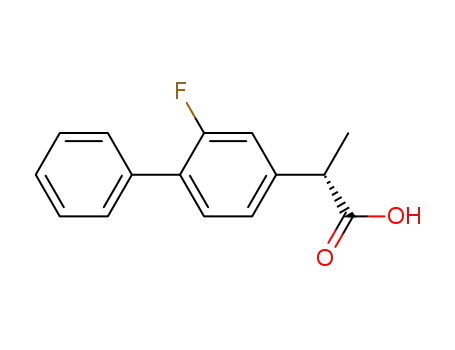
(S)-(+)-flurbiprofen
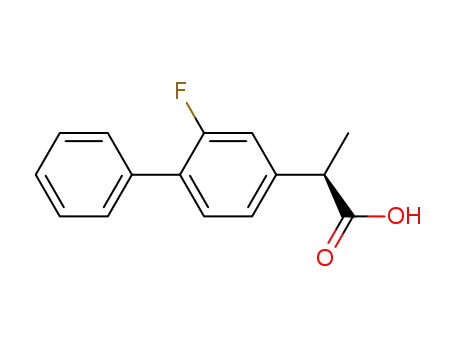
(R)-flurbiprofen
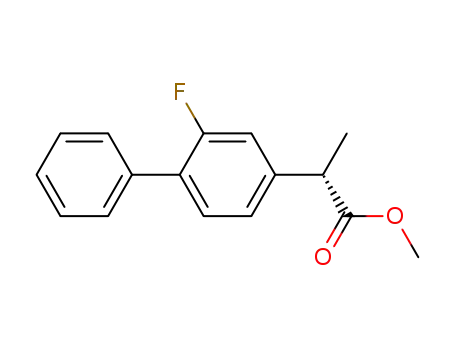
methyl 2-(2-fluoro-biphenyl-4-yl)propionate
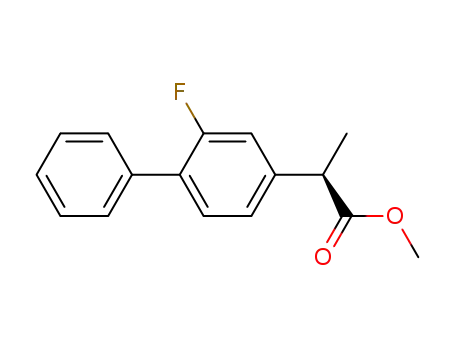
methyl 2-(2-fluoro-biphenyl-4-yl)propionate
