- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Dienogestrel
- 65928-58-7
- C<sub>20</sub>H<sub>25</sub>NO<sub>2</sub>
- 311.424
- Pale yellow solid
Your Location:Home > Products > Biochemical Engineering > Dienogestrel

|
Pharmacodynamics |
Dienogest has progestogenic activity, possibly some antiprogestogenic activity, and has antiandrogenic activity.The medication does not interact with the estrogen receptor, the glucocorticoid receptor, or the mineralocorticoid receptor, and hence has no estrogenic, glucocorticoid, or antimineralocorticoid activity.Because of its relatively high selectivity as a progestogen, dienogest may have favorable safety and tolerability compared to various other progestins. |
|
Features |
Dienogest is a mixed progestogen with dual properties of 19-nortestosterone derivatives and progesterone derivatives; therefore, it combines the pharmacological advantages of natural and synthetic progestins with high progestogenic activity . |
|
Side effects |
Side effects associated with dienogestrel are the same as those expected of a progestogen.They include menstrual irregularities, headaches, nausea, breast tenderness, depression, acne, weight gain, flatulence, and others.Dienogest produces no androgenic side effects and has little effect on metabolic and lipid hemostatic parameters. |
|
Biochem/physiol Actions |
Dienogest is used to treat endometriosis. It might also help to decrease the levels of plasma estradiol by stimulating cell death of granulosa cells in the ovary. |
|
Physical properties |
Dienogestrel is a pale yellow solid. |
|
Definition |
ChEBI: A steroid hormone that is 17beta-hydroxy-3-oxoestra-4,9-diene substituted at position 17 by a cyanomethyl group. Used as an oral contraceptive. |
|
Brand name |
Endometrion (Schering A.G., Germany). |
InChI:InChI=1/C20H25NO2/c1-19-8-6-16-15-5-3-14(22)12-13(15)2-4-17(16)18(19)7-9-20(19,23)10-11-21/h12,17-18,23H,2-10H2,1H3/t17-,18+,19+,20-/m1/s1
The invention relates to a preparation m...
The invention belongs to the technical f...
The invention provides a dienogest compo...
The present invention provides novel pro...
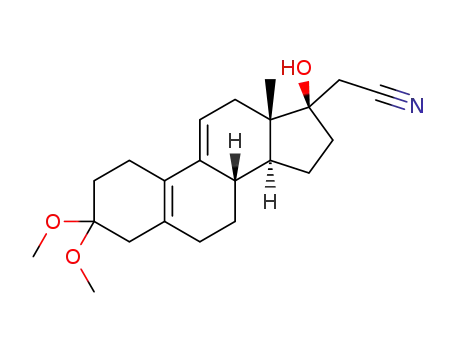
3,3-dimethoxy-17α-cyanomethyl-17β-hydroxy-estra-5(10),9(11)-diene

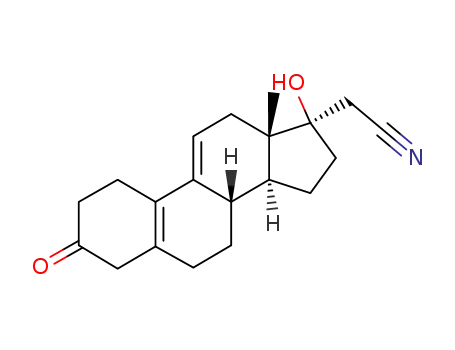
17α-Cyanomethyl-17β-hydroxy-estra-5(10),9(11)-dien-3-on

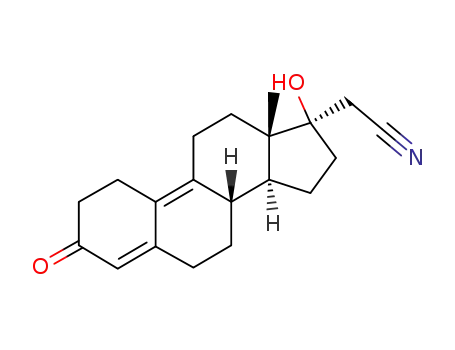
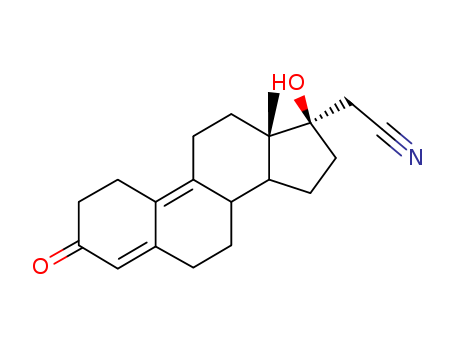
Dienogest
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In water; acetonitrile; at 20 - 30 ℃; Reagent/catalyst; Solvent;
|
74.19 %Chromat. 25.55 %Chromat. |
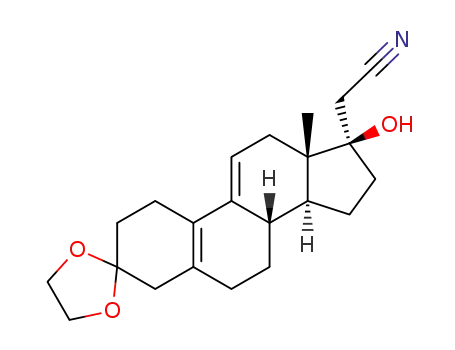
17α-cyanomethyl-17β-hydroxy-5,9-androstadiene-3,17-dione-3,3-ethylene ketal


17α-Cyanomethyl-17β-hydroxy-estra-5(10),9(11)-dien-3-on


Dienogest
| Conditions | Yield |
|---|---|
|
With tetrafluoroboric acid; In water; at 15 - 45 ℃;
|
33.8 g |
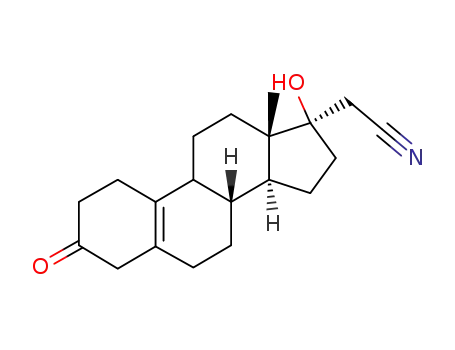
17β-Cyanmethyl-17β-hydroxy-oestr-5(10)-en-3-on
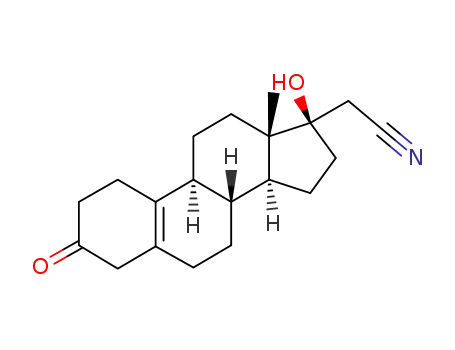
17α-Cyanomethyl-13β-methyl-17β-hydroxy-gon-5(10)-en-3-on
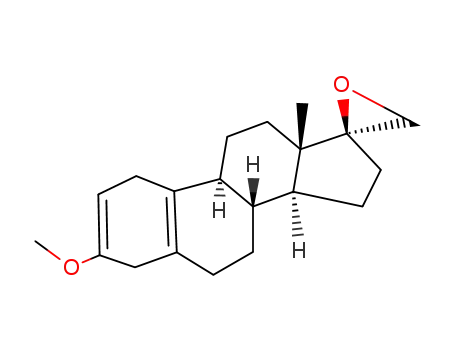
3-methoxy-2,5(10)-estradiene-17S-spiro-oxirane
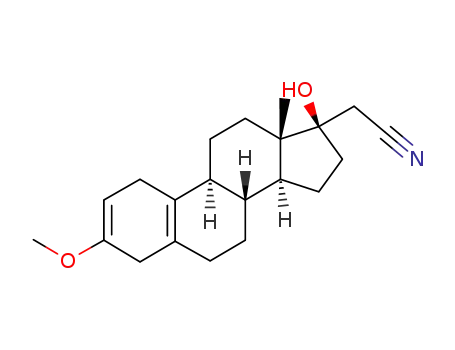
17α-Cyanomethyl-13β-methyl-3-methoxy-gona-2,5(10)-dien-17β-ol
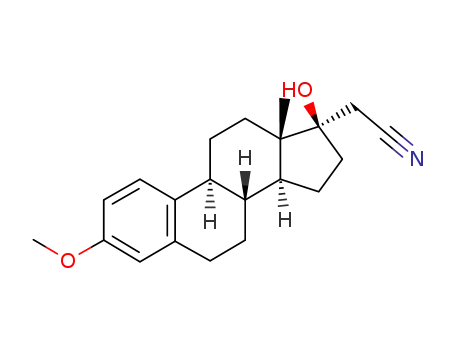
17α-cyanomethyl-1,3,5(10)-estratriene-3,17-diol 3-methyl ether
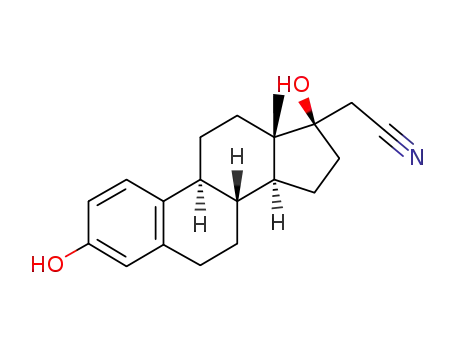
2-((8R,9S,13S,14S,17R)-3,17-dihydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)acetonitrile
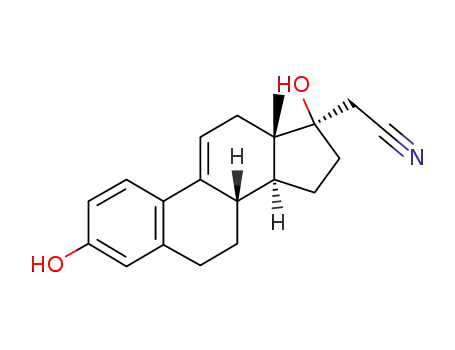
17α-Cyanomethylestra-1,3,5(10),9(11)-tetraen-3,17β-diol
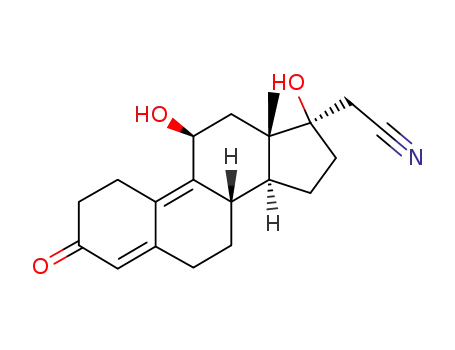
17α-Cyanomethyl-11β,17β-dihydroxy-estra-4,9-dien-3-on
